MEMORIES
2023 has been a demanding exercise for all of us, but also a rewarding one. At all times, we have moved forward with the conviction that our efforts are well supported by a broad set of actors who contribute daily to expanding knowledge about minority pathologies in our country.
Although the past year was marked by the political electoral calendar and a complex global context of war conflicts and economic tensions, we are proud to continue advancing in AELMHU's fundamental lines of action.
At the national level, the different electoral processes held in 2023, both at the regional level and the general elections of July 23, largely conditioned the political and healthcare agenda. The renewal of the teams that make up the different administrations has required, on the part of all agents in the sector, an effort to adapt, always based on the commitment to reinforced collaboration, the continuity of projects and the joint search for effective solutions.
"We are deeply grateful to patient associations, the scientific community, institutions, experts and the media for their full willingness to contribute to the achievement of our shared goals."
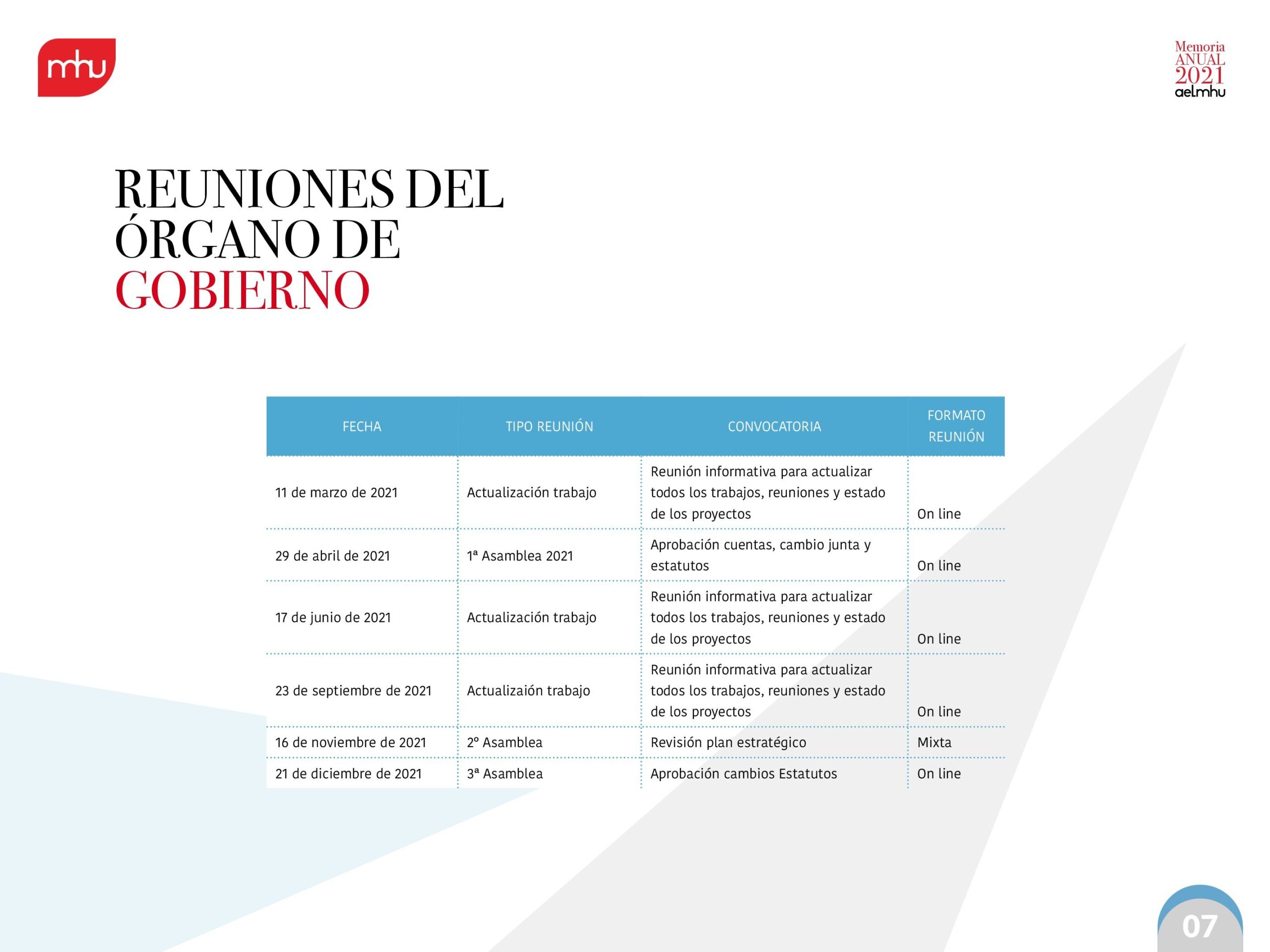
Our working groups have played a key role in this process. Thanks to the involvement of their members, we have broadened our scope to ensure our participation in important discussions on key issues, such as the revision of the Union's general pharmaceutical legislation or the new national model for health technology assessment.
Therefore, on behalf of all the companies that are part of AELMHU, we would like to express our sincere thanks to patient associations, the scientific community, institutions, experts and the media for their full willingness to contribute to the achievement of our shared objectives.
We would like to express our deepest gratitude to each and every one of AELMHU's associates. Your dedication and enthusiasm in joining efforts to promote the different initiatives launched have been fundamental to achieve this year's goals and to continue advancing in our objectives.

2022 has been a complex year for all of us, but it has allowed us to make significant progress in the great challenges of transformation and improvement that we had set for ourselves at AELMHU.
In fact, it has resulted in a clearly positive balance in most areas. Especially because we have made progress in cohesion, support and collaboration with different entities that share with us the objective of improving the quality of life of patients with rare and ultra-rare pathologies and their families.
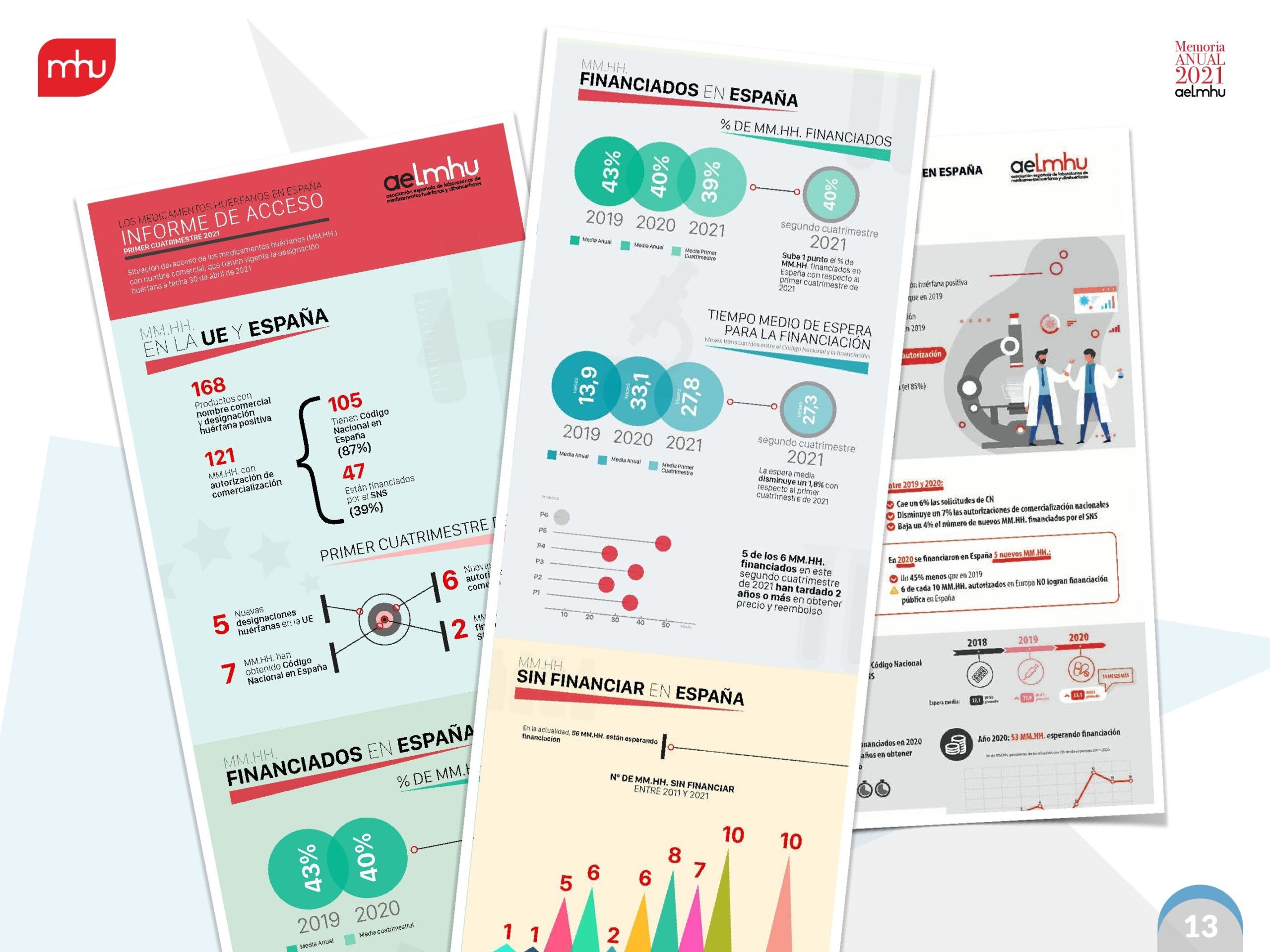
We have managed to consolidate projects and initiatives that have further strengthened our position as a reference entity regarding the evolution of Orphan Drugs in our country. The publication and dissemination of AELMHU's Annual Report on Access to Orphan Drugs in Spain 2022 has allowed us to strengthen our position and our capacity for dialogue with the different experts, agents and key decision-makers.
"On behalf of all the companies that are part of AELMHU, we would like to express our sincere thanks to patient associations, scientific community, institutions, administration and experts for their willingness and collaboration in the objectives we share."
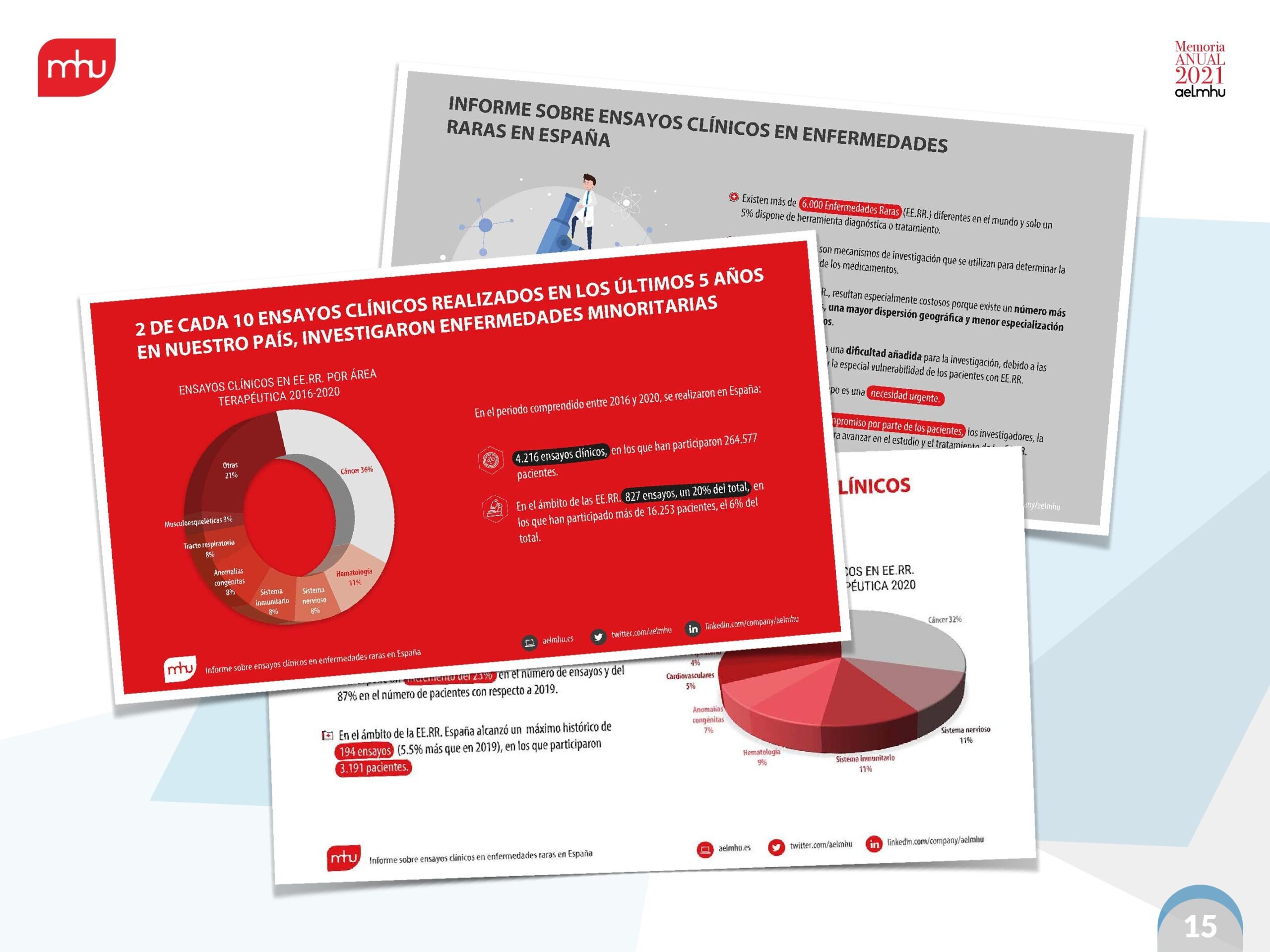
During this year, the results of the research efforts of pharmaceutical and biotechnology companies have enabled a substantial increase in the therapeutic possibilities available in Europe in the field of rare and ultra-rare diseases, as reflected in the data on new approvals from the European Medicines Agency. The definitive irruption of advanced therapies and new developments in different therapeutic areas reinforce a hopeful and positive outlook for patients and relatives of this type of pathologies.
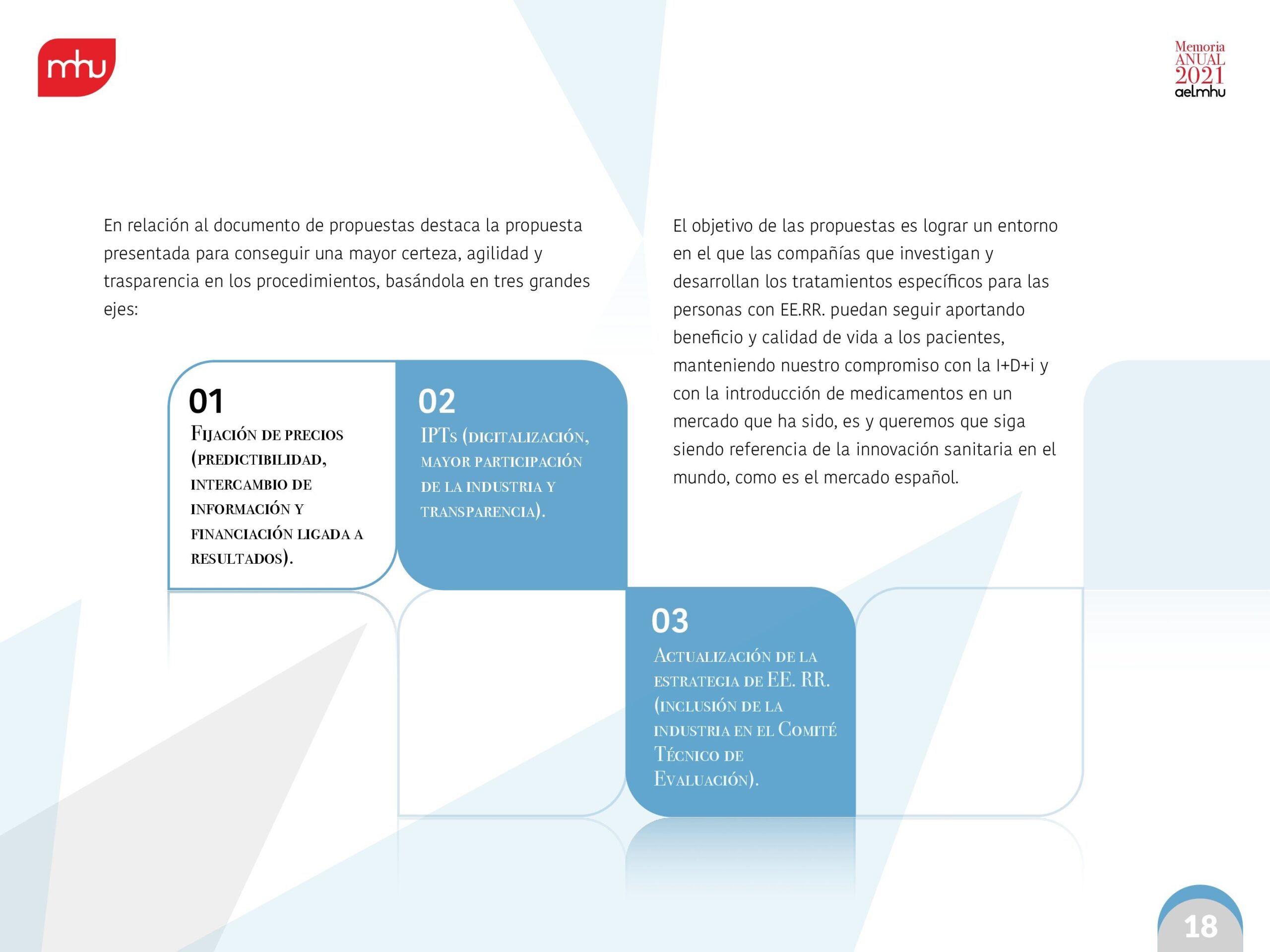
At the national level, AELMHU will continue to provide its willingness, knowledge and collaboration to continue contributing to improving a model that facilitates and speeds up, as much as possible, the availability of these innovations to increase the therapeutic resources of the medical community for these diseases. We will continue to persevere in this effort to continuously improve the therapeutic and social value of orphan drugs in Spain. But, above all, we will continue to becommitted to research and development of innovativetherapies to improve the quality of life of patients.
On behalf of all the companies that are part of AELMHU, we would like to express our sincere thanks to patient associations, the scientific community, institutions, administration and experts for their willingness and collaboration in the objectives we share.

Thanks to all the people who have accompanied us this year both internally and externally. To those who suffer from a rare disease and want to know more about treatments that could change their lives.
To so many researchers, companies and entrepreneurs; brave people who have made the decision to discover a treatment and change the world and seek in our association the warmth and company of those who defend a noble common goal.
For AELMHU, the year 2021 has also been the year in which many of the projects that, with great enthusiasm, we forged in the last stages of 2020 have finally crystallized. A year of growth, momentum, renewal, but also of intense and fruitful work.
2021 was the year of reunion. A gradual reunion between families, friends and colleagues. Reunion in our tasks, in our agendas, plans and work. A reunion, in short, in our shared projects, which had been paralyzed during the hardest phases of the pandemic due to the harsh public health requirements that have modified our way of living, working and developing.
"Thank you to all the people who have accompanied us this year both internally and externally. To those who suffer from a rare disease and want to know more about treatments that could change their lives."
And, of course, to our associates, who have given content and soul to all these projects, and who participate more actively every day in the growth of our association.
To the professionals who every day come face to face with the disease and have decided to be part of the solution, participating in our work to learn about the latest processes and understand the concerns of those who discover and develop them.
To the public decision-makers and administrations, patient associations and scientific societies, who have approached AELMHU to find the truth in the data, who have invited us to participate in their meetings, convinced that this is the best way to transform things and improve the lives of others.

2020 was the year of change. While a global pandemic was shaking social, health and economic structures around the world, AELMHU was celebrating its tenth anniversary. During that year, we faced the challenge of taking the definitive step towards the transformation of the organization.
True to our commitment to give greater visibility to minority diseases, we focused on the lesser-known value of orphan drugs and the importance of all our partner companies, which share a highly innovative profile that has been more relevant than ever during the toughest times of the year.
Faced with such challenges, it was time to undertake a new task: to incorporate new perspectives such as advanced therapies and personalized medicine into our work and to increase the participation of companies in AELMHU's activities, through working groups and the establishment of participative dynamics, which have made the thrust of this association the true desire of the sum of the efforts of all its members.
"It was time to undertake a new task: to incorporate new perspectives such as advanced therapies and personalized medicine into our work and to increase the participation of companies in AELMHU's activity."
The objective was clear: the fight against the pandemic could not forget rare diseases. The efforts of all the agents involved in dealing with these pathologies obliged us to remember that these patients represent a particularly vulnerable group.
Spain has always occupied a leading position in clinical research at the international level. Therefore, the pandemic has strengthened the commitment of Spanish society to guaranteeing public health, clearly demonstrating the direct impact that pharmaceutical innovation has on everyone's life.
In short, 2020 was a decisive year for our Association to position itself as the transforming agent of the reality of access to orphan drugs in our country, and that encourages us to continue working so that all together -the Administration, the industry, the scientific community and patients-, we achieve that no one suffering from a rare disease feels like an orphan in Spain again.