REPORT
on clinical trials on
rare diseases 2023
AELMHU publishes its Annual Report on Clinical Trials in Rare Diseases authorized in Spain during 2023, where the most relevant data on the trials authorized for these pathologies and their evolution in the last five years in our country are analyzed.
In 2023, 190 trials were authorized, 23% of the total number of authorized clinical trials, despite being very complex and heterogeneous areas. A total of 2,884 people participated in these, 34% less than in 2022. In percentage terms, participants in clinical trials for rare diseases accounted for 7% of the total number of participants.
2023


Clinical trials in minority diseases in Spain by 2023
Report
Press release
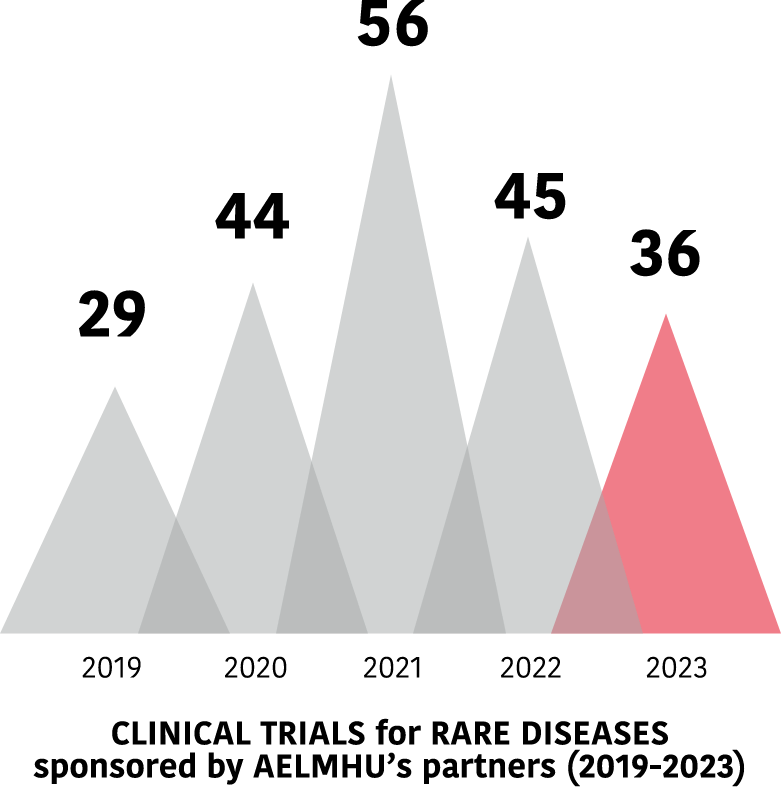
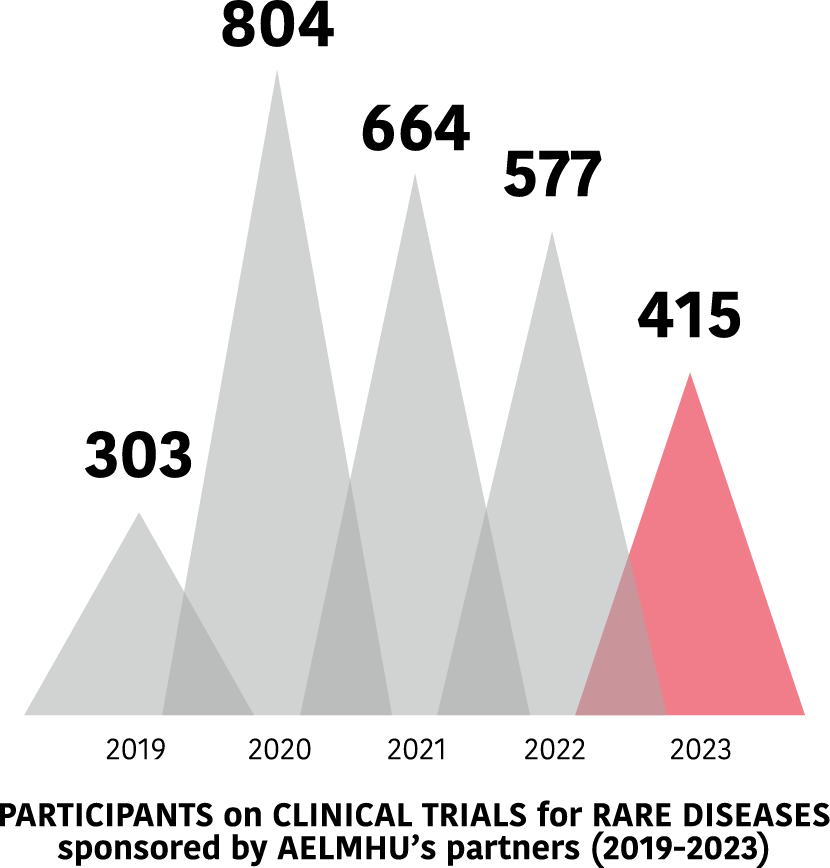
The clinical trials of AELMHU partners
Clinical trials in EE.RR. of AELMHU associates 2019-2023


Evolution of trials between 2019 and 2023.
REPORT
on clinical trials on
rare diseases 2022
AELMHU publishes its Annual Report on Clinical Trials in Rare Diseases authorized in Spain throughout the year 2022. It analyzes the main data on authorized trials and their evolution over the last five years.
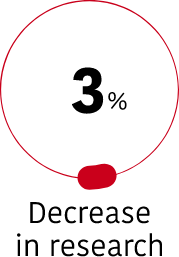
During the year 2022, 233 trials were authorized for research on rare diseases, which shows the great momentum of experimental studies for these pathologies, despite being a very complex and sensitive area and that the total number of clinical trials has decreased compared to the previous year (7%).
Currently, 25% of the trials authorized in Spain are to investigate rare diseases, which represents a percentage growth of 2 points with respect to the year 2021.
2022

Clinical trials in minority diseases in Spain by 2022
Report
Press release
The clinical trials of AELMHU partners
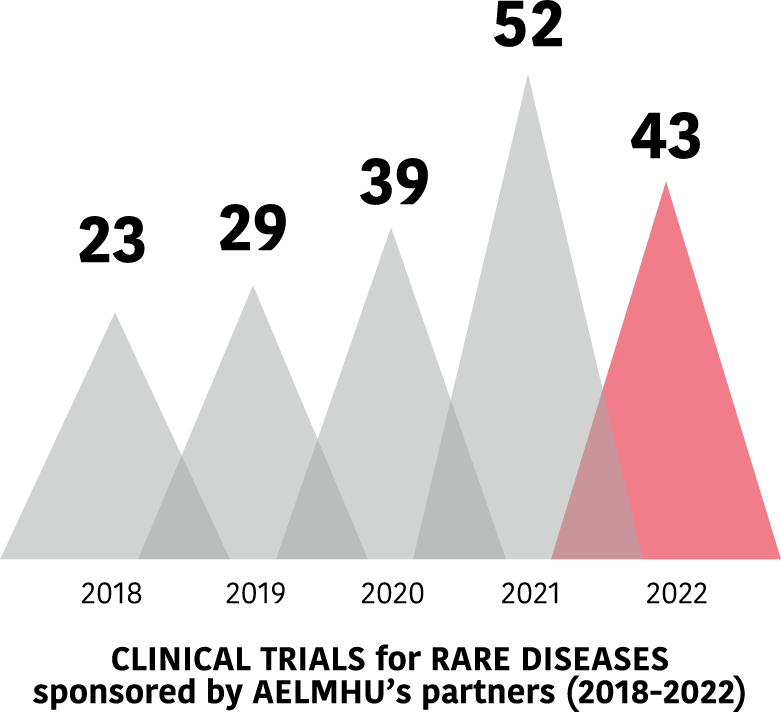
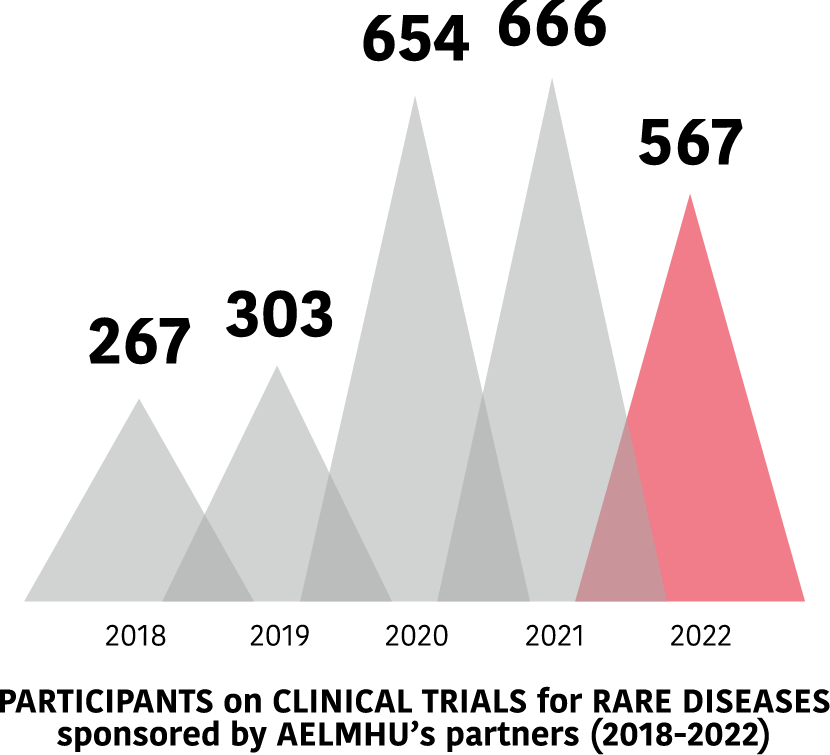
Clinical trials in EE.RR. of AELMHU associates 2018-2022


Evolution of trials between 2018 and 2022.


List of Clinical Trials of AELMHU members
(until December 31, 2022)
Access the complete list of clinical trials that AELMHU members have launched in Spain.
Source: Spanish Registry of Clinical Trials
REPORT
on clinical trials on
rare diseases 2021
Once again, on the occasion of World Clinical Trials Day, AELMHU publishes its Annual Report on Clinical Trials in Rare Diseases in Spain. It analyzes the main data on trials authorized in Spain in 2021 in this area and how these authorizations have evolved over the last 5 years.
During 2021, Spain reached a record number with the launch of 225 trials for the research of rare diseases, which is evidence of the great moment that experimental studies for these pathologies are experiencing, despite being a very complex and sensitive field.
Currently, 23% of the trials authorized in Spain are for research into rare diseases, which represents a percentage growth of 3 points with respect to 2020.
2021
Clinical trials on rare diseases in Spain in 2021
Report
Press release
The clinical trials of AELMHU partners
Clinical trials in EE.RR. of AELMHU associates 2017-2021
Evolution of trials between 2017 and 2021.
List of Clinical Trials of AELMHU members
(until 31 December 2021)
Access the complete list of clinical trials that AELMHU members have launched in Spain.
REPORT
on clinical trials on
rare diseases 2020
On the occasion of World Clinical Trials Day, the Spanish Association of Orphan and Ultra-Orphan Drug Laboratories (AELMHU) presents its Annual Report on Clinical Trials in Rare Diseases in Spain. This study, which analyses the main data on clinical trials authorised in the last 5 years in our country, reveals that during 2020 Spain reached a record number of 194 trials for the research of medicines aimed at the diagnosis and treatment of rare diseases, which shows the great moment that pharmaceutical research is experiencing in its commitment to an area as complex and sensitive as the care of patients with rare diseases (RD).
2020
Clinical trials on rare diseases in Spain in 2020
Report
Press release
The clinical trials of AELMHU partners

Data analysis:
- Compilation of public data on clinical trials on rare diseases promoted by pharmaceutical and biotechnology companies associated with AELMHU, published in the Spanish Registry of Clinical Trials.
- Data analyzed: identifier, active ingredients, phase, status, scientific title, disease investigated, date of authorization, start date, end date in Spain and expected participants in Spain and totals.
AELMHU partners' clinical trials in the USA 2013-2020
Evolution of trials
between 2013 and 2020.
List of Clinical Trials of AELMHU members
(last updated April 2021)
Click here for a complete list of AELMHU member's CCS authorised in Spain until April 2021.